By collecting patient-specific information, boundaries are established for the hand piece so the surgeon can remove the damaged surfaces of the knee, balance the joint, and position the implant with greater precision.
The robotics and AI enhance control of the your unique anatomy to improve outcomes including proper size, implant positioning, soft-tissue balance, and lower limb alignment,.
Please call us to ask any questions:
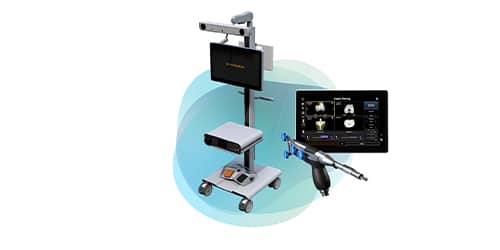
Smith & Nephew NAVIO Robotic-assisted technology used by Dr Hicken and only available at St George Surgical Center and Mesa View Regional Hospital
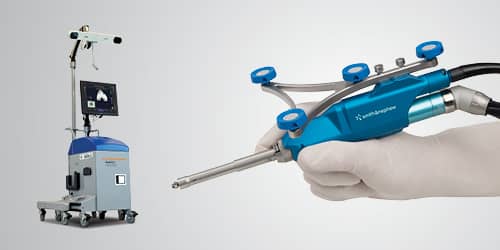
Smith & Nephew CORI Robotic-assisted technology used by Dr Hicken and only available at St George Surgical Center
References
Robotic Orthopaedic Institute St George, LLC
1490 East Foremaster Drive, Ste 260
St George, UT 84790
This website is copyright protected. Plagiarism, piracy, theft, copying application or adaptation, direct or indirect of any content in graphical, video, text or structural form on this page for any reason, in any language, is strictly prohibited, intentional or unintentional actions will result in prosecution at the highest level of DMCA, international copyright infringement and Internet Piracy laws.